显示元素电子壳层结构的原子图
氢


氦

锂

锂是第一种添加了额外电子壳层的元素。记住,价电子位于最外层。电子壳层的填充取决于它们的轨道。第一个轨道(s轨道)只能包含两个电子。
铍

硼

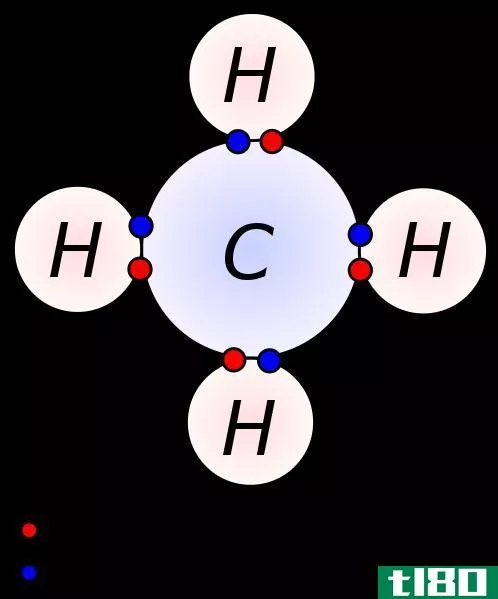
碳

氮

氧气

氟

霓虹的

钠

镁

铝

硅

磷

硫黄

氯

氩

钾

钙

钪

钛

钒

铬

锰

铁

钴

镍

铜

锌

镓

锗

砷

硒

溴

氪

铷

锶

钇

锆

铌

钼

锝

钌

铑

钯

银

镉

铟

锡

锑

碲

碘

氙气

铯

钡剂

镧

铈

镨

钕

镨

钐

铕

钆

铽

镝

钬

铒

铥

镱

镥

铪

钽

钨

铼

锇

铱

白金

金

水星

铊

领导

铋

钋

虾青素

氡

弗朗西姆

镭

锕

钍

精锕

铀

镎

钚

- 发表于 2021-09-11 02:16
- 阅读 ( 330 )
- 分类:化学
你可能感兴趣的文章
价壳(valence shell)和倒数第二壳(penultimate shell)的区别
...与另一个原子的价电子共用,就会形成共价键。 对于s块元素和p块元素,价壳层分别是s轨道和p轨道。但对于过渡元素,价电子也可以存在于内轨道中。这是由于子轨道之间的能量差。例如,锰的原子序数是25。钴的电子构型为[A...
- 发布于 2020-09-26 17:01
- 阅读 ( 867 )
连锁(catenation)和四价(tetravalency)的区别
链结和四价的关键区别在于,链结包括同一化学元素的原子结合形成链状或环状结构,而四价是指形成四个共价键的能力。 由于化学元素碳的特性,它和化学元素碳同时使用链式和四价。碳可以通过共价键与许多碳原子结合...
- 发布于 2020-09-27 17:55
- 阅读 ( 230 )
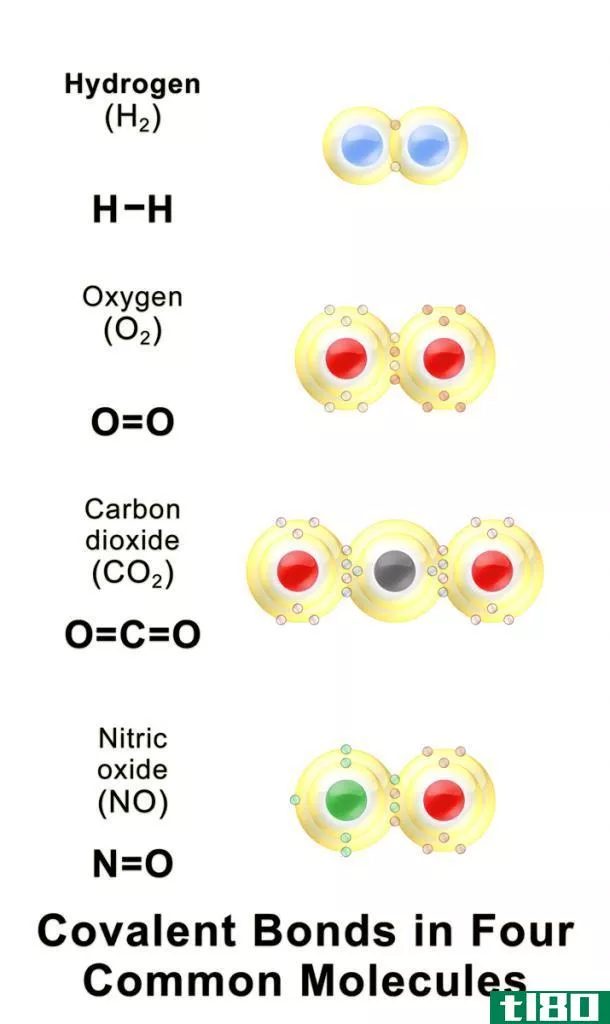
路易斯点符号(lewis dot symbol)和刘易斯结构(lewis structure)的区别
... symbol)? 一个点代表一个电子符号。因此,如果我们需要显示分子中的化学键,那么我们需要使用两个电子作为一对,因为当来自两个不同原子的两个电子彼此配对时,就会形成化学键。此外,我们必须用一对点来表示一对单独...
- 发布于 2020-10-13 17:57
- 阅读 ( 1515 )
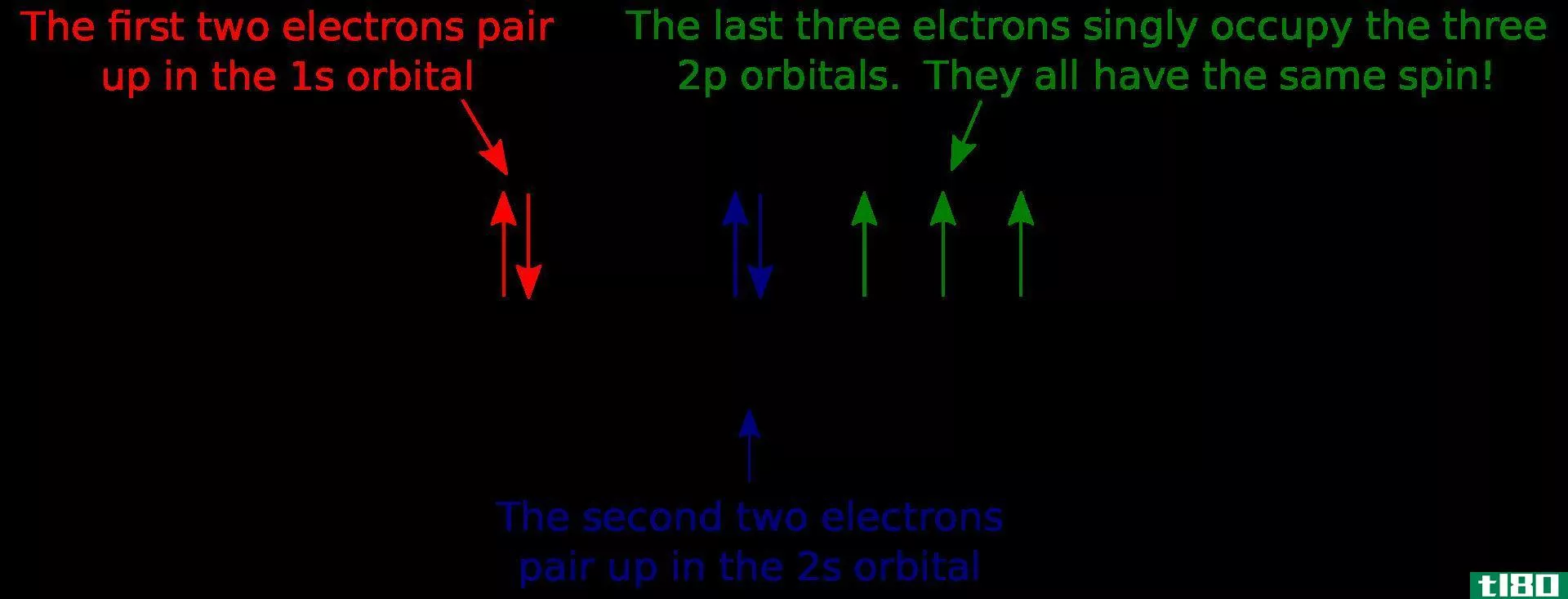
轨道图(orbital diagram)和电子组态(electron configuration)的区别
...用箭头表示电子,表示电子的自旋。但是,电子组态没有显示电子自旋的细节。 轨道图显示了由电子组态给出的电子排列。电子组态给出了电子在整个原子轨道上分布的细节。但是,轨道图也显示了电子的自旋。这就是轨道图...
- 发布于 2020-10-14 00:32
- 阅读 ( 733 )
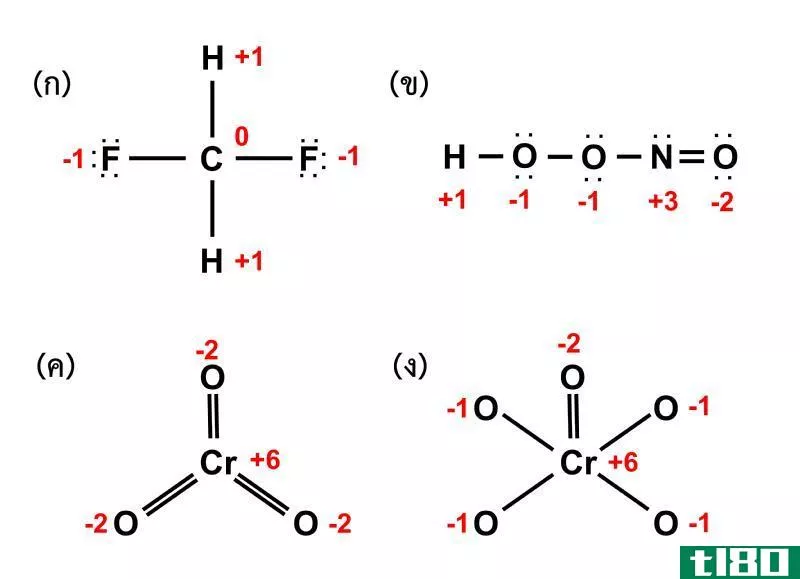
氧化值(oxidation number)和指控(charge)的区别
...数,而电荷是根据原子中电子和质子的总数来确定的。 元素周期表中的不同元素表现出不同的化学和物理特性。当它们结合形成分子时,不同的元素以不同的比例与其他元素结合。在元素之间的大量变化中,最简单和重要的参...
- 发布于 2020-10-15 23:21
- 阅读 ( 379 )
激进的(radical)和价(valency)的区别
...子的化学物种,而价是一个化学概念,它描述了一种化学元素与另一种化学元素结合的能力。 自由基是一种非常活泼的化学物质,因为它有一个不成对的电子。价是一种元素的结合力,特别是用它能置换或结合的氢原子数来衡...
- 发布于 2020-10-17 02:38
- 阅读 ( 400 )
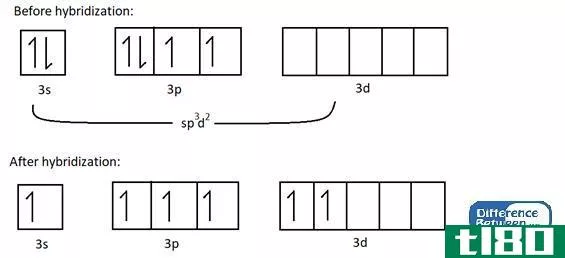
sp3d2型(sp3d2)和d2sp3杂交(d2sp3 hybridization)的区别
...轨道在八面体排列的两个轨道之间有90度角。八面体排列显示了一个正方形平面,其中有四个杂化轨道,其余两个轨道位于该方形平面的上方和下方(垂直于该平面)。 例子 让我们考虑一个例子来理解sp3d2杂交。例:SF6分子具有...
- 发布于 2020-10-19 13:51
- 阅读 ( 722 )
钙(calcium)和镁(magnesium)的区别
...外,钙的原子序数是20,而镁的原子序数是12。 钙和镁是元素周期表第2组中的两种化学元素。虽然它们在同一组中,但它们处于周期表的不同时期,因为钙比镁多了一个电子壳层。因此,它们具有不同的化学和物理性质。 目录 1...
- 发布于 2020-10-22 08:34
- 阅读 ( 668 )
价(valency)和指控(charge)的区别
价态与电荷的关键区别在于,价态表示一种化学元素与另一种化学元素结合的能力,而电荷则表示一种化学元素获得或移除的电子数。 价和电荷是密切相关的术语,因为这两个术语都描述了化学元素的反应性。价是一种元素...
- 发布于 2020-10-23 02:08
- 阅读 ( 346 )
共价性(covalency)和氧化态(oxidation state)的区别
...么获得/释放电子,要么共享电子以填充电子壳层。下表显示了具有不同共价值的化学元素的一些示例。 什么是氧化态(oxidation state)? 一个原子的氧化态是该原子与另一个原子失去、获得或共享的电子数。如果电子丢失或获得,...
- 发布于 2020-10-24 02:21
- 阅读 ( 291 )